
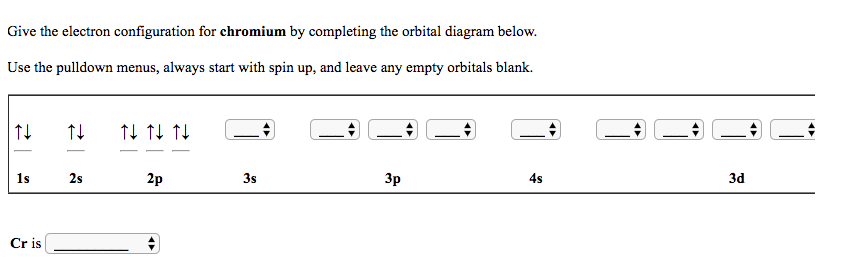
It's a quantum mechanical argument where the parallel-spin electrons can exchange with each other due to their indistinguishability (you can't tell for sure if it's electron 1 that's in orbital 1, or electron 2 that's in orbital 1, etc), reducing the energy of the configuration. It becomes important to incorporate the exchange energy.Įxchange energy is the reduction in energy due to the number of parallel-spin electron pairs in different orbitals. #ul(uarr darr) " " ul(uarr color(white)(darr)) " " ul(uarr color(white)(darr))# When you have something like this with parallel electron spins.

We'd just say that for every electron pair in the same orbital, it adds one #Pi_c# unit of destabilization. To make it easier on us, we can crudely "measure" the repulsion energy with the symbol #Pi_c#. #ul(uarr darr) " " ul(color(white)(uarr darr)) " " ul(color(white)(uarr darr))# is higher in energy than #ul(uarr color(white)(darr)) " " ul(darr color(white)(uarr)) " " ul(color(white)(uarr darr))# Lack of significant reduction of pairing energy overall in comparison to an atom with larger occupied orbitalsĬoulombic repulsion energy is the increased energy due to opposite-spin electron pairing, in a context where there are only two electrons of nearly-degenerate energies.Minimization of coulombic repulsion energy.It's also worth mentioning that these reasons are after-the-fact chromium doesn't know the reasons we come up with the reasons just have to be, well, reasonable. However, for chromium, it's the significant reason. :)Ī lot of people want to say that it's because a "half-filled subshell" increases stability, which is a reason, but not necessarily the only reason. Pertinent valence orbitals NOT close enough in energy for electron pairing to be stabilized enough by large orbital sizeĭISCLAIMER: Long answer, but it's a complicated issue, so.More electrons with parallel spins in separate orbitals.Less electrons paired in the same orbital.


 0 kommentar(er)
0 kommentar(er)
